| Catalogue number | C108645 |
| Chemical name | Stevenleaf |
| CAS Number | 80321-63-7 |
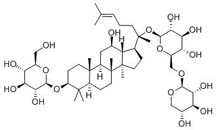
| Synonyms | (2R,3R,4S,5S,6R)-2-[[(10R,12S,14R)-17-[(2R)-2-[[(2S,3R,4R,5S,6R)-6-[[(2S,3R,4S,5R)-4,5-dihydroxy-3-methoxy-2-oxanyl]oxymethyl]-3,4,5-trihydroxy-2-oxanyl]oxy]-6-methylhept-5-en-2-yl]-12-hydroxy-4,4,10,14-tetramethyl-1,2,3,5,6,7,8,9,11,12,13,15,16,17-tetrad |
| Molecular Weight | C41H68O13 |
| Formula | 768.9 |
| Purity | 98% |
| Physical Description | White powder |
| Solvent | Chloroform, Dichloromethane,DMSO |
| Storage | Stored at 2-8°C, Protected from air and light, refrigerate or freeze |
| Applications | Gypenosides (Gyp), components of Gynostemma pentaphyllum Makino, were found to induce suppression of human tongue squamous cell carcinoma SCC4 cell growth and induce apoptosis in response to overexpression of reactive oxygen species, calcium (Ca+2) and to decrease mitochondrial membrane potential in vitro. In this study, the effect of Gyp on cell migration and invasion of human tongue SCC4 cells was examined. SCC4 cells treated in vitro with Gyp migrated and invaded less than cells treated with phosphate-buffered saline (PBS) as a control. Gyp inhibited migration and invasion by down-regulating the production of RAS, NFÎB, COX2, ERK1/2 and MMP-9 relative to PBS only. These results show that Gyp inhibits invasion and migration of human tongue SCC4 cells by down-regulating proteins associated with these processes, resulting in reduced metastasis.
|
| References | 1. Phytochemistry, 1983, 22(6), 1473-1478. 2. Studies in Plant Science, 1999, 6, 77-82. 3. J. Nat. Prod., 2003, 66(7), 922-927. 4. Anticancer Research, 2008, 28(2A), 1093-1099. 5. Zhongguo Zhong Yao Za Zhi, 1996, 21(9), 562-564. 6. Zhongguo Zhong Yao Za Zhi, 1996, 21(5), 299-302, 320. |
| Guestbook |
| C262180 | Verbascoside | $72.00/20mg |
| C101411 | Ganoderol B | $294.00/5mg |
| C108539 | 1,3-Dicaffeoylquinic acid | $182.00/20mg |
| C108004 | Rubusoside | $72.00/20mg |
| C104837 | Zederone | $298.00/5mg |
| Size | Price(USD) | Discount |
| 5mg | Inquiry | N/A |
| 10mg | Inquiry | N/A |
| 25mg | Inquiry | N/A |
To place an order, please provide the following information.
1) Your name and telephone number
2) Purchase order number
3) Product number, package size, description, and quantity
4) Shipping and billing addresses
Sent to your order to our email: info@coompo.com