| Catalogue number | C108528 |
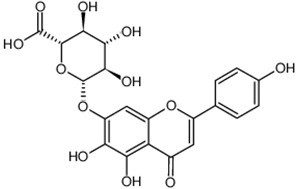
| Chemical name | Scutellarin |
| CAS Number | 27740-01-8 |
| Synonyms | (2S,3S,4S,5R,6S)-6-[[5,6-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-1-benzopyran-7-yl]oxy]-3,4,5-trihydroxy-2-oxanecarboxylic acid |
| Molecular Weight | C21H18O12 |
| Formula | 462.3 |
| Purity | 98% |
| Physical Description | Yellow powder |
| Solvent | Chloroform, Dichloromethane,DMSO |
| Storage | Stored at 2-8°C, Protected from air and light, refrigerate or freeze |
| Applications | Scutellarin has been shown to induce apoptosis of ovarian and breast tumor cells in vitro.
|
| References | 1. J. Asian Nat. Prod. Res., 2003, 5(4), 249-256. 2. Biomacromolecules, 2004, 5(5), 1956-1961. 3. Die Pharmazie - An International Journal of Pharmaceutical Sciences, 2006, 61(8), 660-663(4). 4. Biomacromolecules,, 2004, 5(5), 1956-1961. 5. Life Sciences, 2004, 74(24), 2959-2973. 6. Biochemical and Biophysical Research Communications, 2005, 334(3), 812-816. 7. Pharmacological Research, 2005, 51(3), 205-210. |
| Guestbook |
| C257649 | Tangeretin | $56.00/20mg |
| C108534 | Isochlorogenic acid A | $110.00/20mg |
| C104426 | Chrysin 6-C-glucoside 8-C-arabinoside | $358.00/5mg |
| C108036 | Gomisin N | $230.00/20mg |
| C104530 | Ginsenoside Rh4 | $294.00/10mg |
| Size | Price(USD) | Discount |
| 5mg | Inquiry | N/A |
| 10mg | Inquiry | N/A |
| 25mg | Inquiry | N/A |
To place an order, please provide the following information.
1) Your name and telephone number
2) Purchase order number
3) Product number, package size, description, and quantity
4) Shipping and billing addresses
Sent to your order to our email: info@coompo.com