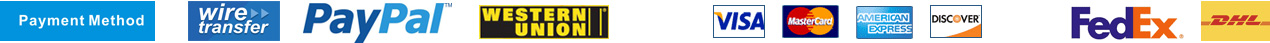| Catalogue number | C108856 |
| Chemical name | Protopanaxatriol |
| CAS Number | 1453-93-6 |
| Synonyms | (3S,5S,6S,8S,9S,10R,12R,13S,14R)-17-[(2R)-2-hydroxy-6-methylhept-5-en-2-yl]-4,4,8,10,14-pentamethyl-2,3,5,6,7,9,11,12,13,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthrene-3,6,12-triol |
| Molecular Weight | C30H52O4 |
| Formula | 476.7 |
| Purity | 98% |
| Physical Description | White powder |
| Solvent | Chloroform, Dichloromethane,DMSO |
| Storage | Stored at 2-8°C, Protected from air and light, refrigerate or freeze |
| Applications | Ginsenosides from the protopanaxatriol group but not from the protopanaxadiol group enhance the release of nitric oxide from endothelial cells and may contribute to the beneficial effect of ginseng on the cardiovascular system.
Protopanaxatriol ginsenosides has a chemosensitizing effect on P-glycoprotein -mediated multidrug resistance cells by increasing the intracellular accumulation of drugs through direct interaction with P-glycoprotein at the azidopine site. In addition, Protopanaxatriol ginsenosides may have a beneficial effect on cancer chemotherapy with respect to the possibility of long-term use without the concern of P-glycoprotein activation.
Peroxisome proliferator-activated receptor gamma , a member of the nuclear receptor of ligand-activated transcription factors, regulates the expression of key genes involved in lipid and glucose metabolism or adipocyte differentiation. Ligands for this receptor have emerged as potent insulin sensitizers used in the treatment of Type2 diabetes. Using a GAL-4/PPARgamma transactivation assay, 20(S)-protopanaxatriol (PPT), one of the ginsenoside metabolites, was found to increase PPARgamma-transactivation activity dose-dependently with similar activity as troglitazone, a well-known PPARgamma agonist. PPT enhanced adipogenesis by increasing the expression of PPARgamma target genes such as aP2, LPL and PEPCK. Furthermore, PPT significantly increased expression of glucose transporter 4 (GLUT4). These results indicate that PPT can be developed as a PPARgamma agonist for the improvement of insulin resistance associated with diabetes. |
| References | 1. Magnetic Resonance in Chemistry, 2002, 40(7), 483-488. 2. J. Nat. Prod., 2008, 71(3), 478-481. 3. Life Sciences, 1995, 56(19), 1577-1586. 4. Planta Med., 2003, 69(3), 235-240. 5. Fitoterapia, 2010, 81(8), 1079-1087. 6. Biological and Pharmaceutical Bulletin, 2006, 29(1), 110-113. |
| Guestbook |
| C104280 | 3-O-Feruloylquinic acid | $422.00/5mg |
| C108848 | Ginsenoside Rh3 | $134.00/5mg |
| C108612 | Glycitin | $56.00/20mg |
| C104529 | Ginsenoside Rg5 | $134.00/10mg |
| C107861 | Trifolirhizin | $126.00/20mg |
| Size | Price(USD) | Discount |
| 5mg | Inquiry | N/A |
| 10mg | Inquiry | N/A |
| 25mg | Inquiry | N/A |
To place an order, please provide the following information.
1) Your name and telephone number
2) Purchase order number
3) Product number, package size, description, and quantity
4) Shipping and billing addresses
Sent to your order to our email: info@coompo.com