| Catalogue number | C108620 |
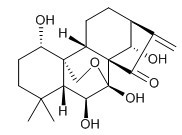
| Chemical name | Oridonin |
| CAS Number | 28957-04-2 |
| Synonyms | (1S,4AR,5S,6S,14S)-1,5,6,14-Tetrahydroxy-4,4-dimethyl-8-methylenedecahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]naphthalen-7(8H)-one |
| Molecular Weight | C20H28O6 |
| Formula | 364.4 |
| Purity | 98% |
| Physical Description | Yellow cryst. |
| Solvent | Chloroform, Dichloromethane,DMSO |
| Storage | Stored at 2-8°C, Protected from air and light, refrigerate or freeze |
| Applications | Oridonin inhibited growth of multiple myeloma (MM; U266, RPMI8226), acute lymphoblastic T-cell leukemia (Jurkat), and adult T-cell leukemia (MT-1) cells with an effective dose that inhibited 50% of target cells (ED50) ranging from 0.75 to 2.7 μg/ml. oridonin inhibited nuclear factor-κB (NF-κB) DNA-binding activity in these cells as measured by luciferase reporter gene, ELISA-based, and electrophoretic mobility shift assays. Oridonin also blocked tumor necrosis factor-α– and lipopolysaccharide-stimulated NF-κB activity in Jurkat cells as well as RAW264.7 murine macrophages. Oridonin did not affect survival of normal lymphoid cells from healthy volunteers. Taken together, oridonin might be useful as adjunctive therapy for individuals with lymphoid malignancies, including the lethal disease adult T-cell leukemia.Oridonin has been proved to possess remarkable anticancer activity, in addition to its potential in antiinflammation and antibacteria.
|
| References | 1. J. Sep. Sci., 2006, 29(2), 314-318. 2. Molecules, 2011, 16, 7949-7957. 3. Journal of Asian Natural Products Research, 2008, 10(1), 77-78. 4. Mol Cancer Ther., 2005, 4, 578. 5. Cell Research, 2007, 17, 274-276. |
| Guestbook |
| C104160 | 8-Prenylnaringenin | $334.00/5mg |
| C108812 | Ginsenoside Compound K | $126.00/20mg |
| C234475 | Echinacoside | $72.00/20mg |
| C104102 | Acetylshikonin | $334.00/20mg |
| C101167 | Arteannuin B | $230.00/20mg |
| Size | Price(USD) | Discount |
| 5mg | Inquiry | N/A |
| 10mg | Inquiry | N/A |
| 25mg | Inquiry | N/A |
To place an order, please provide the following information.
1) Your name and telephone number
2) Purchase order number
3) Product number, package size, description, and quantity
4) Shipping and billing addresses
Sent to your order to our email: info@coompo.com