| Catalogue number | C108918 |
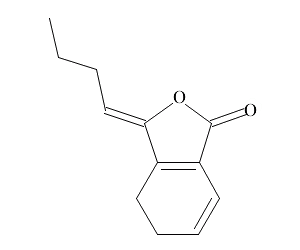
| Chemical name | Ligustilide |
| CAS Number | 4431-01-0 |
| Synonyms | (3Z)-3-butylidene-4,5-dihydroisobenzofuran-1-one |
| Molecular Weight | C12H14O2 |
| Formula | 190.2 |
| Purity | 98% |
| Physical Description | Oil |
| Solvent | Chloroform, Dichloromethane,DMSO |
| Storage | Stored at 2-8°C, Protected from air and light, refrigerate or freeze |
| Applications | Ligustilide was tested for its anti-inflammatory activities in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages. We found that ligustilide strongly inhibitis the induction of LPS-induced inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) at both the mRNA and protein levels in a dose-dependent manner. The transcriptional activity of nuclear factor kappa B (NF-B) was also down-regulated in a concentration-dependent manner. Further study revealed that ligustilide inhibited the phosphorylation and subsequent degradation of IBα, an inhibitor protein of NF-B. In addition, ligustilide inhibited the phosphorylation of p38 mitogen-activated protein kinase (p38 MAPK), extracellular signal-regulated kinase (ERK) and c-Jun NH2-terminal kinase (JNK) in a dose-dependent manner. Taken together, these data suggest that ligustilide can exert its antiinflammatory effects by regulating the NF-B and MAPK signal pathways.
|
| References | 1. Archives of Pharmacal Research, 2012, 35(4), 723-732. 2. Brain Research, 2006, 1102(1), 145-153. 3. Journal of Ethnopharmacology, 2007, 112(1), 211-214. |
| Guestbook |
| C108536 | Isochlorogenic acid C | $166.00/20mg |
| C104615 | Kushenol C | $468.00/5mg |
| C107871 | Neochlorogenic acid | $150.00/20mg |
| C108532 | Chlorogenic acid | $40.00/20mg |
| C101410 | Ganoderol A | $474.00/5mg |
| Size | Price(USD) | Discount |
| 5mg | Inquiry | N/A |
| 10mg | Inquiry | N/A |
| 25mg | Inquiry | N/A |
To place an order, please provide the following information.
1) Your name and telephone number
2) Purchase order number
3) Product number, package size, description, and quantity
4) Shipping and billing addresses
Sent to your order to our email: info@coompo.com