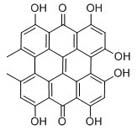
| Synonyms | 1,3,4,6,8,13-Hexahydroxy-10,11-di-methylphenanthro(1,10,9,8,opqra)perylene-7,14-dione; 4,5,7,4’,5’,7’-Hexahydroxy-2,2’-dimethylnaphthodianthrone |
| Applications | Hypericin is a naphthodianthrone, a red-colored anthraquinone-derivative. Hypericin is believed to act as an antibiotic, antiviral and non-specific kinase inhibitor. Hypericin may inhibit the action of the enzyme dopamine β-hydroxylase, leading to increased dopamine levels, although thus possibly decreasing norepinephrine and epinephrine.
In the last three decades, hypericin has also become the subject of intensive biochemical research and is proving to be a multifunctional agent in drug and medicinal applications. Recent studies report antidepressive, antineoplastic, antitumor and antiviral activities of hypericin; intriguing information even if confirmation of data is incomplete and mechanisms of these activities still remain largely unexplained. In other contemporary studies, screening hypericin for inhibitory effects on various pharmaceutically important enzymes such as MAO (monoaminoxidase), PKC (protein kinase C), dopamine-beta-hydroxylase, reverse transcriptase, telomerase and CYP (cytochrome P450), has yielded results supporting therapeutic potential. Research of hypericin and its effect on GABA-activated (gamma amino butyric acid) currents and NMDA (N-methyl-D-aspartat) receptors also indicate the therapeutic potential of this substance whereby new insights in stroke research (apoplexy) are expected. Also in the relatively newly established fields of medical photochemistry and photobiology, intensive research reveals hypericin to be a promising novel therapeutic and diagnostic agent in treatment and detection of cancer (photodynamic activation of free radical production). Hypericin is not new to the research community, but it is achieving a new and promising status as an effective agent in medical diagnostic and therapeutic applications. New, although controversial data, over the recent years dictate further research, re-evaluation and discussion of this substance.
It was initially believed that the anti-depressant pharmacological activity of hypericin was due to inhibition of monoamine oxidase enzyme. The crude extract of Hypericum is a weak inhibitor of MAO-A and MAO-B. Isolated hypericin does not display this activity, but does have some affinity for NMDA receptors. This points in the direction that other constituents are responsible for the MAOI effect. The current belief is that the mechanism of antidepressant activity is due to the inhibition of reuptake of certain neurotransmitters.
Hypericin, a powerful naturally occurring photosensitizer, is found in Hypericum perforatum plants, commonly known as St. John's wort. In recent years increased interest in hypericin as a potential clinical anticancer agent has arisen since several studies established its powerful in vivo and in vitro antineoplastic activity upon irradiation. Investigations of the molecular mechanisms underlying hypericin photocytotoxicity in cancer cells have revealed that this photosensitizer can induce both apoptosis and necrosis in a concentration and light dose-dependent fashion. Moreover, Photodynamic therapy (PDT) with hypericin results in the activation of multiple pathways that can either promote or counteract the cell death program.
|
| References | 1. Monatshefte für Chemie / Chemical Monthly, 1992, 123(10), 931-938.
2. Monatshefte für Chemie / Chemical Monthly, 1993, 124(6-7), 751-761.
3. J. Am. Chem. Soc., 1999, 121(35), 7979-7988.
4. The International Journal of Biochemistry & Cell Biology, 2002, 34(3), 221-241.
5. Pharmacopsychiatry, 1997, 30(2), 94-101.
6. Curr Pharm Des., 2005, 11(2), 233-253.
|