| Catalogue number |
C107836 |
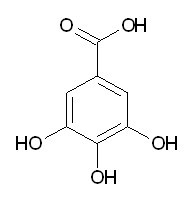
| Chemical name | Gallic acid |
| CAS Number | 149-91-7 |
| Synonyms | 3,4,5-trihydroxybenzoic acid |
| Molecular Weight | C7H6O5 |
| Formula | 170.1 |
| Purity | 98% |
| Physical Description | Powder |
| Solvent | Chloroform, Dichloromethane,DMSO |
| Storage | Stored at 2-8°C, Protected from air and light, refrigerate or freeze |
| Applications | Antineoplastic agent, astringent. Possesses bacteriostatic activity.
Gallic acid, a naturally occurring plant phenol with antioxidative activity, was found to induce cell death in promyelocytic leukemia HL-60RG cells, although many antioxidants are well known to protect the cell from oxidative stress. Morphological and biochemical studies indicated that the gallic acid-induced cell death is apoptosis. Flow cytometric analysis revealed that the apoptosis was not triggered at a specific phase of the cell cycle and that 2 h exposure of gallic acid to HL-60RG cells was enough to induce apoptosis. The inhibitory assay suggested that gallic acid-induced cell death was mediated by reactive oxygen species such as hydrogen peroxide, superoxide anion in addition to Ca2+ ion, calmodulin-dependent enzymes. Structure-activity analysis suggests that gallic acid induces apoptosis in HL-60RG cells, depending on its distinctive feature derived from the structure but not on its antioxidative activity.
Gallic acid was found to possess anti-inflammatory activity towards zymosan-induced acute food pad swelling in mice. In vitro studies on the mode of action of gallic acid revealed that this compound interferes with the functioning of polymorphonuclear leukocytes (PMNs). Scavenging of superoxide anions, inhibition of myeloperoxidase release and activity as well as a possible interference with the assembly of active NADPH-oxidase may account for the inhibition of inflammatory process by gallic acid. Structure-activity relationship analysis showed that the o-dihydroxy group of gallic acid is important for the inhibitory activity in vitro.
|
| References | 1. Aldrich Library of 13C and 1H FT NMR Spectra, 1992, 2, 1142C; 1143A; 1147C; 1148B; 1261B; 1261C; 1352C; 1539C
2. J. Agric. Food Chem., 1993, 41 (11), 1880-1885.
3. Biochemical and Biophysical Research Communications, 1994, 204(2), 898-904.
4. J. Agric. Food Chem., 2004, 52 (2), 255-260
5. Planta Med., 1992, 58(6), 499-504.
|
| Guestbook |
|
The packaging of the product may have turned upside down during transportation, resulting in the product adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
| Size | Price(USD) | Discount |
| 5mg | Inquiry | N/A |
| 10mg | Inquiry | N/A |
| 25mg | Inquiry | N/A |
Orders can be placed by Emails. All orders received will be shipped in the next day if the stock is available.
To place an order, please provide the following information.
1) Your name and telephone number
2) Purchase order number
3) Product number, package size, description, and quantity
4) Shipping and billing addresses
Sent to your order to our email: info@coompo.com
If you have any questions about discounts or dealer discount, please send us a message. We will be glad to help.