| Catalogue number | C108529 |
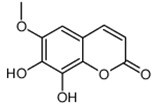
| Chemical name | Fraxetin |
| CAS Number | 574-84-5 |
| Synonyms | 7,8-dihydroxy-6-methoxy-1-benzopyran-2-one |
| Molecular Weight | C10H8O5 |
| Formula | 208.1 |
| Purity | 98% |
| Physical Description | Cryst. |
| Solvent | Chloroform, Dichloromethane,DMSO |
| Storage | Stored at 2-8°C, Protected from air and light, refrigerate or freeze |
| Applications | Incubation of cells with the fraxetin led to a significant elevation dose-dependent of cellular GSH and this was accompanied by a marked protection against rotenone-mediated toxicity, which was also significantly reversed in the cells with buthionine sulfoximine (BSO) co-treatment. Taken together, suggested that intracellular GSH appeared to be an important factor in fraxetin-mediated cytoprotection against rotenone-toxicity in SH-SY5Y cells. Fraxetin at 10-100 muM inhibited the formation of ROS, cytochrome c release, activation of caspase-3 and 9, and suppressed the up-regulation of Bax, whereas no significant change occurred in Bcl-2 levels. Our results indicated that the anti-oxidative and anti-apoptotic properties render this natural compound potentially protective against rotenone-induced cytotoxicity.
|
| References | 1. Chemistry of Natural Compounds, 1982, 18(6), 745-746. 2. Journal of Pharmacy and Pharmacology, 1997, 49(1), 49-52. 3. Biol. Pharm. Bull., 2006, 29(1), 119-124. 4. Neurosci Res., 2005, 53(1), 48-56. 5. European Journal of Pharmacology, 2003, 472(1-2), 81-87. |
| Guestbook |
| C104615 | Kushenol C | $468.00/5mg |
| C108539 | 1,3-Dicaffeoylquinic acid | $182.00/20mg |
| C257538 | Syringin | $80.00/20mg |
| C103985 | Ganoderiol F | $704.00/10mg |
| C262180 | Verbascoside | $72.00/20mg |
| Size | Price(USD) | Discount |
| 5mg | Inquiry | N/A |
| 10mg | Inquiry | N/A |
| 25mg | Inquiry | N/A |
To place an order, please provide the following information.
1) Your name and telephone number
2) Purchase order number
3) Product number, package size, description, and quantity
4) Shipping and billing addresses
Sent to your order to our email: info@coompo.com