| Catalogue number | C108810 |
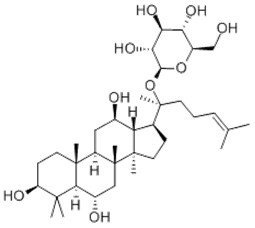
| Chemical name | Ginsenoside F1 |
| CAS Number | 53963-43-2 |
| Synonyms | (2R,3S,4R,5R,6S)-2-(hydroxymethyl)-6-[(2R)-6-methyl-2-[(6R,10R,12S,13R,14R,17S)-3,6,12-trihydroxy-4,4,10,14,17-pentamethyl-2,3,5,6,7,8,9,11,12,13,15,16-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl]hept-5-en-2-yl]oxyoxane-3,4,5-triol |
| Molecular Weight | C36H62O9 |
| Formula | 638.8 |
| Purity | 98% |
| Physical Description | White powder |
| Solvent | Chloroform, Dichloromethane,DMSO |
| Storage | Stored at 2-8°C, Protected from air and light, refrigerate or freeze |
| Applications | Ginsenoside F1, an enzymatically modified derivative of ginsenoside Rg1, against ultraviolet-B-induced damage in human HaCaT keratinocytes. Ginsenoside F1 significantly reduced ultraviolet-B-induced cell death and protected HaCaT cells from apoptosis caused by ultraviolet B irradiation. Furthermore, ginsenoside F1 prevented ultraviolet-B-induced cleavage of poly(ADP-ribose) polymerase in HaCaT cells. In search of the molecular mechanism responsible for the antiapoptotic effect of ginsenoside F1, we find that protection from ultraviolet-B-induced apoptosis is tightly correlated with ginsenoside-F1-mediated inhibition of ultraviolet-B-induced downregulation of Bcl-2 and Brn-3a expression.
|
| References | 1. Journal of Investigative Dermatology, 2003, 121, 607-613. 2. Planta Med., 2006, 72(2), 126-131. 3. Chemical and Pharmaceutical Bulletin, 2003, 51(4), 404-408. |
| Guestbook |
| C101250 | Chrysosplenol D | $322.00/5mg |
| C104171 | Curzerenone | $374.00/5mg |
| C108612 | Glycitin | $56.00/20mg |
| C104533 | Glychionide A | $334.00/5mg |
| C104080 | Ganoderenic acid D | $381.00/10mg |
| Size | Price(USD) | Discount |
| 5mg | Inquiry | N/A |
| 10mg | Inquiry | N/A |
| 25mg | Inquiry | N/A |
To place an order, please provide the following information.
1) Your name and telephone number
2) Purchase order number
3) Product number, package size, description, and quantity
4) Shipping and billing addresses
Sent to your order to our email: info@coompo.com